Brought to you in partnership with Mermaid Medical Group.
User-innovation is not a new concept within healthcare and the medical device sector, and neither is inventive physicians. However, having an idea and subsequently seeking to commercialize it, is for most medical doctors a rather unknown path. In this brief article, we summarize the case of how the well-known invention of a guidewire and its use has further progressed into the newly launched self-steering guidewire for SFA-access, the Marsman SpeedWire®.
Generally speaking, it is reckoned that there are two types of medical innovators, two types of medical innovations, and two types of medical innovation providers.
Concerning inventors the world has seen cases of both inventive physicists and engineers, e.g. Röntgen who invented x-ray imaging, but also medical doctors such as Gianturco who become known for inventing embolization coils. When referring to medical innovations, we suggest that there needs to be distinguished between two concepts of innovations: (1) fundamentally new ideas, such as the computed tomography which was invented by Hounsfield, and (2) incremental innovations, often defined as being slight or significant changes to previous inventions. An example of the latter was seen in how DSA in 1979 followed the analogue subtraction angiography, which was originally invented by the Dutch radiologist Ziedses des Plantes back in 1935.
Lastly, medical innovations are often commercialized and diffused towards professional users by either existing MedTech companies or by new companies, which may be established by medical doctors. Companies of the second type appear truly interesting as these are founded by the professional medical doctors and innovators themselves. As we have experienced, there are several well-known cases where medical doctors have established companies with the aim of sharing their innovations with medical colleagues. An example of the latter could be found in the establishment of Le Maitre Vascular, originally founded by the vascular surgeon George Le Maitre. This is an excellent example of a vascular surgeon who was not satisfied with the commercially available devices to cut valves in peripheral veins, and hence he invented a device for cutting valves without the requirement of direct vision.
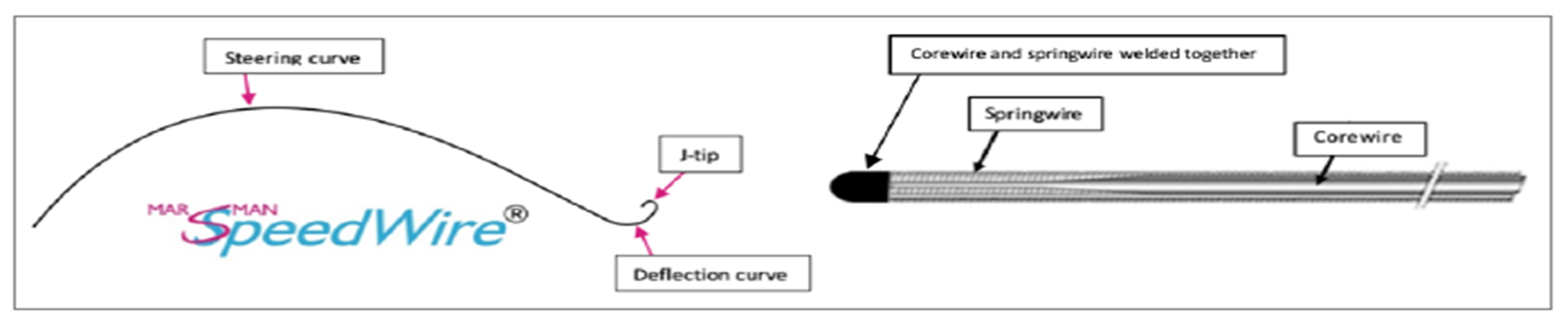
Considering the elaborated medical innovation typology, we argue that the newly launched Marsman SpeedWire® should be perceived as an incremental medical innovation based on the fundamental guidewire idea of the Swedish radiologist Sven-Ivar Seldinger. Nowadays, Seldinger’s guidewire technique is used to safely guide a wide array of devices into the human body, ranging from introduction sheaths to cutting balloons, and from implants of stents to heart valves. As a result, there is no doubt that the inventor of the Marsman SpeedWire®, Interventional radiologist Pieter Marsman, stands on Seldinger’s shoulders with his innovation of a self-steering guidewire.
 Even though we today can look at the case of the Marsman SpeedWire® as a success in regard to bringing an idea to market, it is worth noting that the process of having a prototype of an invention, and subsequently see it being used by medical colleagues, did not unfold without the encountering of challenges. When asking Mr Marsman, it was clear that certain obstacles arose in the work of getting established medical device companies to translate his idea into a real product. Some of these multinationals did either not acknowledge the applicability of his idea, and some even raised concerns regarding the self-steering guidewire’s commercial potential. Therefore, Mr Marsman had to take a path that for him as a clinician was unfamiliar – setting up his own company. First, he applied for patenting of his idea which was issued in Europe and the US in 2014. Subsequently, he made and tested prototypes in his kitchen lab. Satisfied with the results of his tests, he presented the findings at the RSNA annual meeting in Chicago, 2016.
Even though we today can look at the case of the Marsman SpeedWire® as a success in regard to bringing an idea to market, it is worth noting that the process of having a prototype of an invention, and subsequently see it being used by medical colleagues, did not unfold without the encountering of challenges. When asking Mr Marsman, it was clear that certain obstacles arose in the work of getting established medical device companies to translate his idea into a real product. Some of these multinationals did either not acknowledge the applicability of his idea, and some even raised concerns regarding the self-steering guidewire’s commercial potential. Therefore, Mr Marsman had to take a path that for him as a clinician was unfamiliar – setting up his own company. First, he applied for patenting of his idea which was issued in Europe and the US in 2014. Subsequently, he made and tested prototypes in his kitchen lab. Satisfied with the results of his tests, he presented the findings at the RSNA annual meeting in Chicago, 2016.
When reflecting upon the Marsman SpeedWire®, there is no doubt that it is a MD4MD device; made by an MD for his fellow MDs, blossoming out of collegial interaction between inventor and clinicians. The clinical use of this unique guidewire for SFA-access in a significant number of patients, combined with feedback from colleagues, was crucial for the development of more advanced guidewire. This convoluted but very interesting innovation journey led to the CE-certification of the Marsman SpeedWire® in 2018.
With great joy, we argue that due to the guidewire’s advanced engineering and capability, one might say that the Marsman SpeedWire® blindly finds and enters the SFA.
This news story has been sponsored by the companies concerned and does not represent the views or opinions of RAD Magazine.